Urigen
Pharmaceuticals
305 East High Street
Suite 7
Bound Brook,
NJ 08805
United States
ph:
732-369-5911
 URG101
URG101
URG101 is combination of lidocaine and heparin instilled directly into the bladder via catheter. Lidocaine provides immediate symptom relief, while high dose heparin (also a GAG) helps to mechanically repair the damaged bladder lining.
The individual components of this combination therapy, Lidocaine HCl and heparin sodium, are FDA-approved products for use as a local anesthetic and an anticoagulant, respectively. It was demonstrated that when these components were mixed together and buffered by the addition of sodium bicarbonate, the resultant solution reduced symptoms of pelvic pain and urgency upon instillation into a subject’s bladder. Most subjects exhibit both an acute benefit as well as sustained benefit from single and multiple treatments. This multimodal therapy achieves acute benefit for subjects who have no standard acute treatment for pelvic pain and urgency of bladder origin.
Clinical Efficacy
Our Phase II data shows the greatest separation from placebo ever published in IC/BPS
Key Findings
- Immediate relief with first URG101 dose
- Many patients experienced > 24hr symptom relief following only one dose
- Significant reduction of daytime symptoms
- Many patients experienced fewer night time voiding episodes
URG101 Phase II Pharmacodynamic Study
% Change in Pain Over Time, n=18 patients
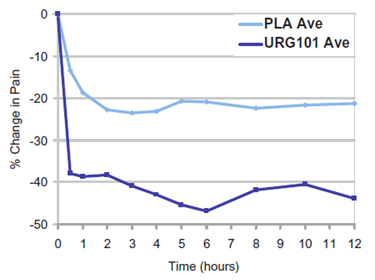
Source: Parsons et at. J Sex Med 2012;9:207–212
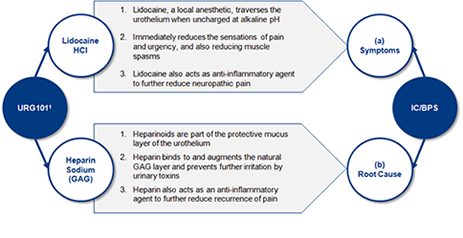
Mechanism of Action
Intravesical URG101 has a dual action mechanism:
a) reducing pain and b) repairing the GAG layer
URG101
Clinical
Trials
Ended |
|
Completed |
Study of URG101 in Painful Bladder Syndrome and Interstitial Cystitis |
Completed |
Copyright 2020 Urigen. All rights reserved.
Urigen Pharmaceuticals
305 East High Street
Suite 7
Bound Brook, NJ 08805
United States
ph:
732-369-5911
